Understanding The Concept Of Molecular Geometry
Have you ever wondered about the three-dimensional arrangement of atoms in a molecule and how it affects its properties? This is where the concept of molecular geometry comes into play. Understanding the molecular geometry of a compound is crucial in predicting its behavior, reactivity, and physical properties. In this article, we will delve into the world of molecular geometry, exploring its significance, determining factors, and real-life applications.
What is the Molecular Geometry?
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule and the associated bond angles. It provides a structural insight into the shape of a molecule, which in turn influences its polarity, reactivity, and overall behavior. The study of molecular geometry is essential in various fields such as chemistry, biochemistry, and pharmacology, as it helps in understanding the structure-function relationship of molecules.
The Significance of Molecular Geometry
Why does molecular geometry matter?
The significance of molecular geometry lies in its ability to determine the physical and chemical properties of a molecule. By understanding the spatial arrangement of atoms and the resulting shape of a molecule, scientists can make predictions about its behavior, including its polarity, reactivity, and interactions with other molecules. This knowledge is valuable in fields such as drug design, material science, and environmental studies, where understanding the structure of compounds is crucial.
Factors Influencing Molecular Geometry
What determines the molecular geometry of a compound?
The molecular geometry of a compound is influenced by several factors, including the number of bonding electron pairs and the number of lone pairs around the central atom. Additionally, the presence of multiple bonds and the repulsion between electron pairs also play a significant role in determining the overall shape of a molecule. Understanding these factors is essential in predicting the molecular geometry of different compounds and molecules.
Real-life Applications of Molecular Geometry
How is the concept of molecular geometry applied in real life?
The understanding of molecular geometry has numerous practical applications. For example, in the field of drug design, knowledge of the three-dimensional structure of molecules is crucial in developing effective pharmaceuticals. Similarly, in environmental studies, the prediction of molecular shapes helps in understanding the behavior of pollutants and their impact on the ecosystem. Furthermore, in the field of nanotechnology, the control of molecular geometry is essential in designing new materials with specific properties.
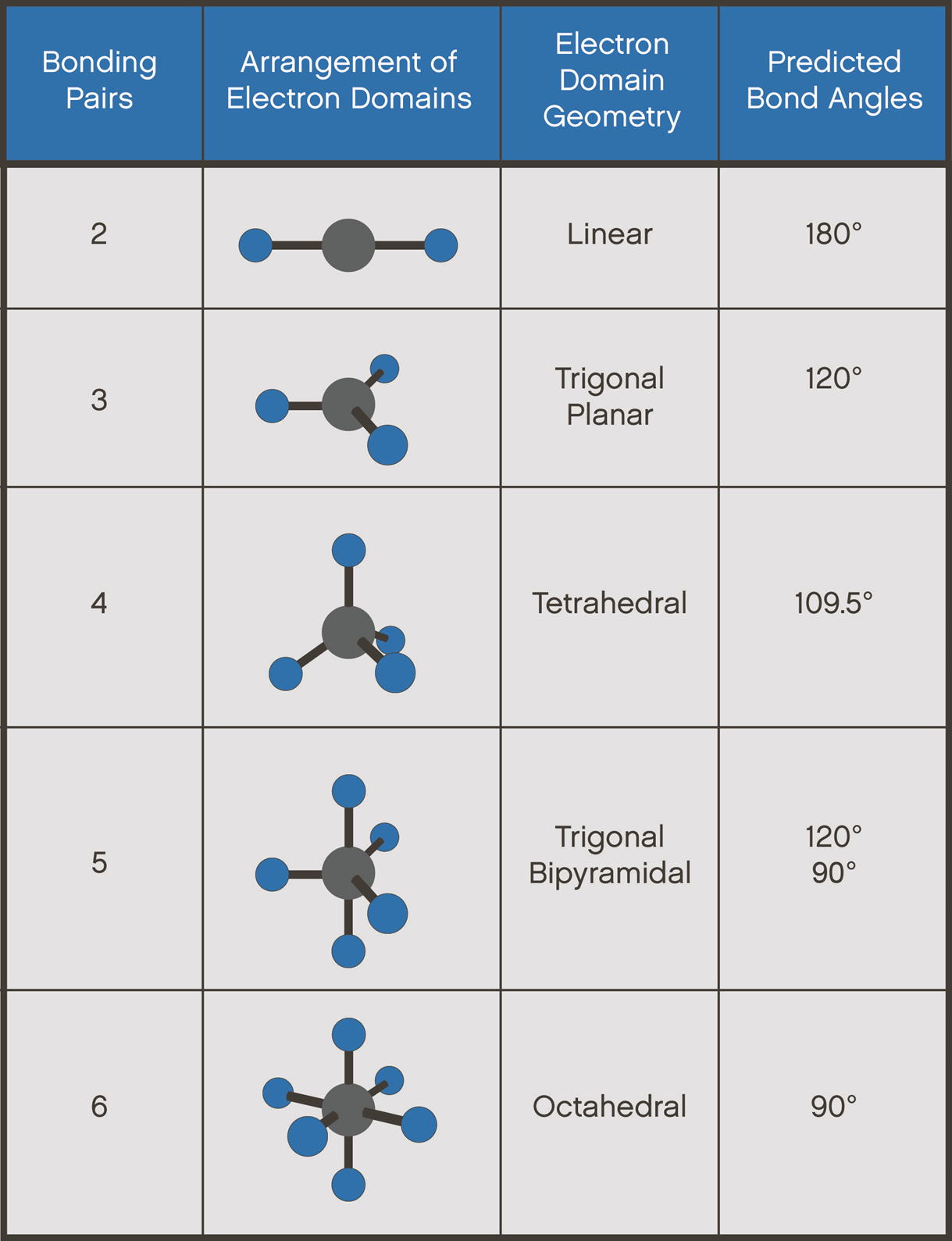
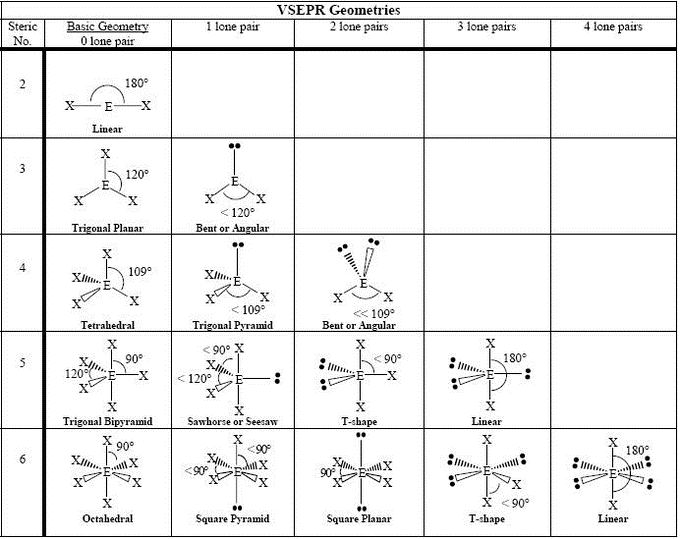
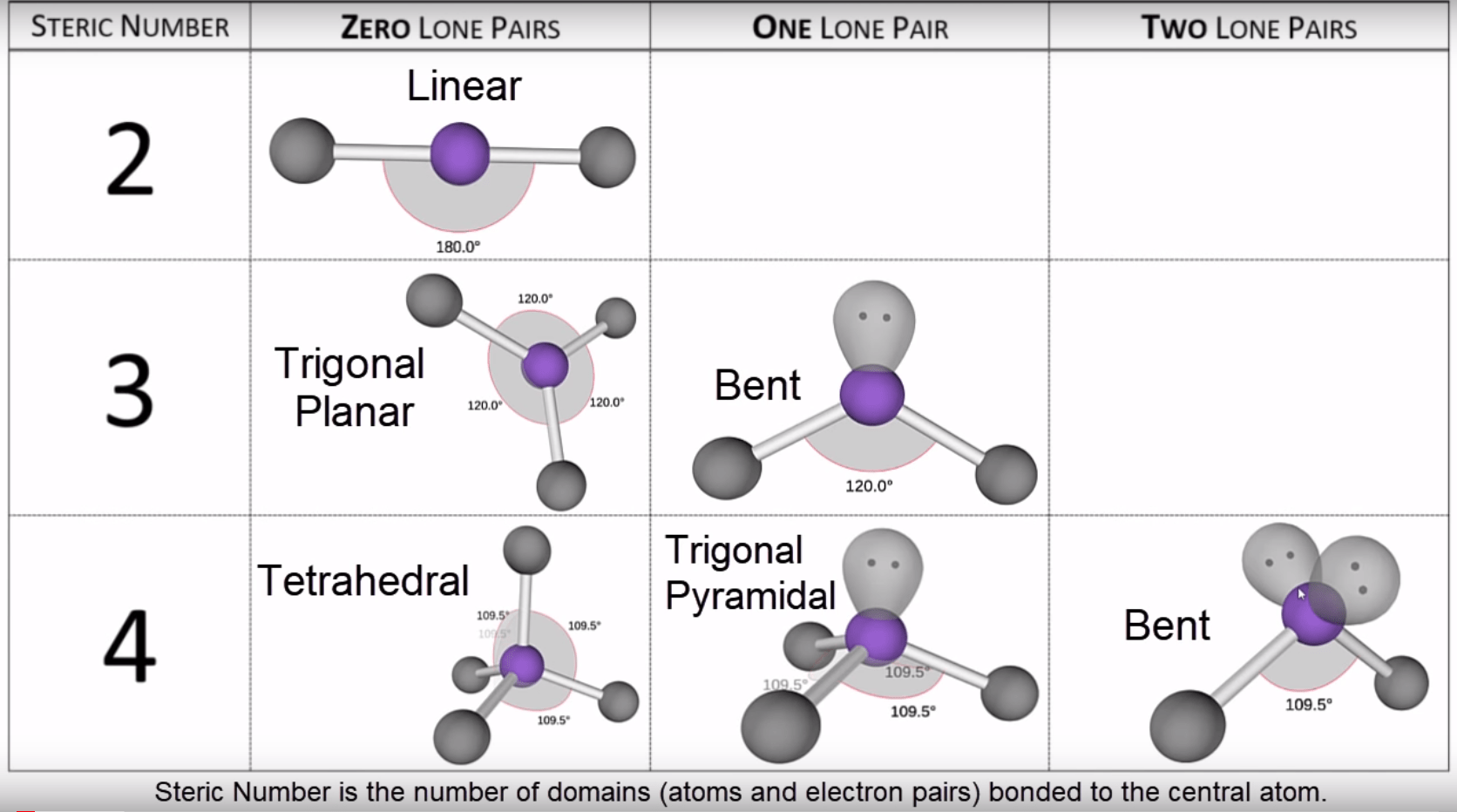
Common Molecular Geometries
What are some common molecular geometries?
There are several common molecular geometries that are observed in chemical compounds. These include linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral geometries, among others. Each of these geometries has specific bond angles and shapes, which determine the overall properties and behavior of the molecules.
Experimental Techniques for Determining Molecular Geometry
How do scientists experimentally determine the molecular geometry of a compound?
Several experimental techniques are employed to determine the molecular geometry of a compound. These include X-ray crystallography, electron diffraction, and nuclear magnetic resonance (NMR) spectroscopy, among others. These techniques provide valuable insights into the three-dimensional arrangement of atoms in a molecule, allowing scientists to elucidate its structure and properties.
Importance of Molecular Geometry in Chemical Bonding
How does molecular geometry influence chemical bonding?
The molecular geometry of a compound is intimately linked to its chemical bonding behavior. The arrangement of atoms and electron pairs in a molecule determines the type of bonds present, as well as the overall polarity of the molecule. This, in turn, influences its interactions with other molecules and its reactivity in chemical reactions. Understanding the role of molecular geometry in chemical bonding is essential in elucidating the behavior of different compounds.
In conclusion, the concept of molecular geometry plays a crucial role in understanding the structure and behavior of molecules. By delving into the three-dimensional arrangement of atoms in a compound, scientists can make valuable predictions about its properties and reactivity. The real-life applications of molecular geometry are vast, spanning from drug design to environmental studies. Overall, molecular geometry is a fundamental concept in the field of chemistry, with far-reaching implications in various scientific disciplines.
Actress Bambi Allen Wikipedia Age
Luis Strohmeier Wife Age Wikipedia
The Smiths S Sheila Take: Unraveling The Mystery


ncG1vNJzZminpJrFunrApqpsZpSetKrAwKWmnJ2Ro8CxrcKeqmebn6J8uLTArWSiq12ptaZ5zKijnpuloa6zecaepqadpKfGb7TTpqM%3D